- 77% of patients treated with Levulan PDT experienced 75% clearance of AK lesions vs 23% of vehicle-treated patients (p<0.001)
- 83% of the patients treated with Levulan PDT had 75% clearance of face lesions and 60% of the patients had 75% clearance of scalp lesions.
- 66% of patients treated with Levulan PDT experienced 100% clearance of AK lesions vs 13% of vehicle-treated patients (p<0.001)
- 70% of the patients treated with Levulan PDT had 100% clearance of face lesions and 55% of the patients had 100% clearance of scalp lesions.
Second treatment with Levulan PDT improved complete response rates*
Of the 56 patients who received a second treatment, 43% of them achieved 100% clearance.
Percent of patients who had a 75-100% response at 8 weeks

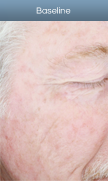
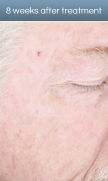
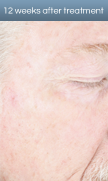
AK lesion response in a patient treated with Levulan PDT
Results after one Levulan PDT treatment
*Results from two identically designed, Phase III studies. These were multi-center, blinded, active treatment-controlled, randomized, uneven parallel group, two-arm studies. A total of 243 patients were randomized at a 3 to 1 Levulan to vehicle ratio. Patients returned for follow-up visits 24 hours after BLU-U light treatment and at weeks 1, 4 and 8. Those patients who were not complete responders at week 8 had retreatment of the persistent target lesions at week 8. All patients returned at 12 weeks after the initial treatment.
Indication
Levulan® Kerastick® (aminolevulinic acid HCl) for Topical Solution, 20% plus blue light illumination using the BLU-U® Blue Light Photodynamic Therapy Illuminator is indicated for the treatment of minimally to moderately thick actinic keratoses of the face or scalp.
Important Risk Information
Application of Levulan® Kerastick® should involve either scalp or face lesions, but not both simultaneously. Levulan® Kerastick® should not be applied to the periorbital area or allowed to contact ocular or mucosal surfaces. Excessive irritation may be experienced if this product is applied under occlusion.
Contraindicated in patients with cutaneous photosensitivity at wavelengths of 400-450 nm, porphyria, or known allergies to porphyrins, and in patients with known sensitivity to any of the components of the Levulan Kerastick for Topical Solution.
Levulan® Kerastick® has not been tested on patients with inherited or acquired coagulation defects. It is possible that concomitant use of other known photosensitizing agents might increase the photosensitivity reaction of actinic keratoses treated with the Levulan® Kerastick®.
Patients should avoid exposure of the photosensitive treatment sites to sunlight or prolonged or intense light for at least 40 hours after application of the Levulan Kerastick Topical Solution. Exposure may result in a stinging and/or burning sensation and may cause erythema or edema of the lesions. Patients should protect treated lesions from the sun by wearing a wide-brimmed hat or similar head covering of light-opaque material. Sunscreens will not protect against photosensitivity reactions caused by visible light.
Most common adverse events including stinging and/or burning, itching, erythema, and edema were observed in all clinical studies. Severe stinging and/or burning at one or more lesions being treated was reported by at least 50% of patients at some time during treatment. However, less than 3% of patients discontinued light treatment due to stinging and/or burning.
During light treatment, both patients and medical personnel should be provided with blue blocking protective eyewear, as specified in the BLU-U operating instructions to minimize ocular exposure.
For additional important safety information, please see full prescribing information.




